Projects
Pollutants to Products: Leveraging Metal-Ligand Cooperation for Small Molecule Activation
Carter Wynne, BSc
Undergraduate Summer Researcher
Project: Base metal pincer complexes featuring boranes in the secondary coordination sphere for nitrile hydroboration.
Ali Nasoudi, 3rd-Year Undergraduate (UPEI)
Undergraduate Summer Researcher
Project: Catalytic esterification of carboxylic acids and alcohols promoted by ligand peripheral silane moiety.
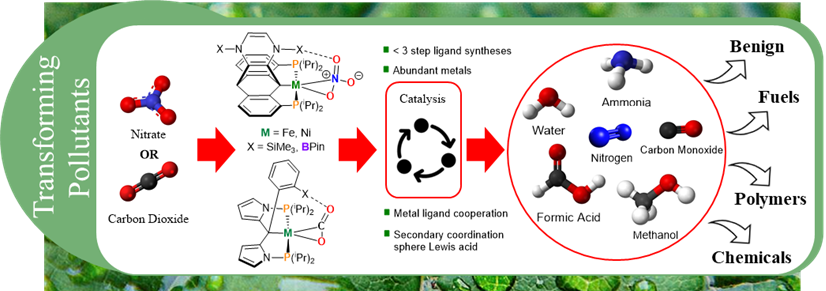
Tridentate pincer ligands have proven highly successful in supporting base metal catalysts due to their readily tunable steric and electronic properties. Metal-ligand cooperation, in which both metal and ligand partake in small molecule activation, has become a popular method for the transformation of challenging molecules to value-added materials. Recently, organometallic research has turned its attention to the impacts of Lewis acid and base incorporation into the ligand secondary coordination sphere and their ability to promote metal ligand cooperation in the activation of challenging substrates.
This research focuses on two primary goals:
1) Introduction of Lewis acidic and basic moieties into the secondary coordination sphere of pincer ligands to promote metal ligand cooperation in the activation of small molecules, stereoselective transformations, and sustainable catalysis.
2) Leveraging redox active moieties in the pincer framework to stabilize first-row transition metal mechanistic intermediates and improve catalytic turnover.
Lead Researchers:
Carter Wynne, Ali Nasoudi, Marissa L. Clapson
Key References:
1. Meiere, S. H.; Ding, F.; Friedman, L. A.; Sabat, M.; Harman, W. D. Dihapto Coordination of Carboxylic Acid Derivatives with an Asymmetric Rhenium π-Base: A New Mechanism for Amide Isomerization? J. Am. Chem. Soc. 2002, 124 (45), 13506–13512.
2. Clapson, M. L.; Dilinaer, A. D.; Lanaque, T. C. Q.; Zurakowski, J. A.; Austen, B. J. H.; Drover, M. W. Ynone Co-Coordination at a Nickel Borane Complex: An Assessment of Secondary Coordination Sphere Effects. Inorg. Chem. 2024, 63, 14, 6184–6191.
3. Zhu, G.; Wang, L.; Sun, H.; Li, X. Formation of PCP Pincer Cobalt Complexes with Cobaltacyclopropane Moieties via Double Csp3–H Bond Activation. RSC Adv. 2015, 5 (25), 19402–19408.
4. Clapson, M. L.; Sharma, H.; Zurakowski, J. A.; Drover, M. W. Cooperative Nitrile Coordination Using Nickel and a Boron-Containing Secondary Coordination Sphere. Chem. Eur. J. 2023. e202203763.
5. Nerush, A.; Vogt, M.; Gellrich, U.; Leitus, G.; Ben-David, Y.; Milstein, D. Template Catalysis by Metal–Ligand Cooperation. C–C Bond Formation via Conjugate Addition of Non-Activated Nitriles under Mild, Base-Free Conditions Catalyzed by a Manganese Pincer Complex. J. Am. Chem. Soc. 2016, 138 (22), 6985–6997.
6. Lu, Z.; Williams, T. J. A Dual Site Catalyst for Mild, Selective Nitrile Reduction. Chemical Communications 2014, 50 (40), 5391–5393.
7. Clapson, M. L.; Kirkland, J.; Piers, W. E.; Ess, D. H.; Gelfand, B.; Lin, J. Carbene Character in a Series of Neutral PCcarbeneP Cobalt(I) Complexes: Radical Carbenes versus Nucleophilic Carbenes. Organometallics 2022, 41, 3, 235–245.
Water Soluble Base Metal Capsule Complexes for Cross Coupling Catalysis and Molecular Sensing
Stephany Graham, 2nd-Year Undergraduate (RDC)
Undergraduate Summer Researcher
Project: Water Soluble Base Metal Cyclodextrin Capsule Complexes for Cross Coupling Catalysis.
Sam MacDonald, 4th-Year Undergraduate (UPEI)
Honours Student Researcher
Project: Water Soluble Nickel Cyclodextrin Capsule Complexes for Cross Coupling Catalysis.
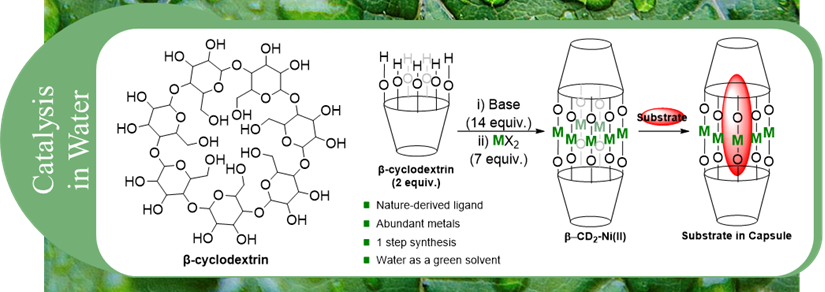
The incorporation of metals into well-defined macro-ligand structures can allow for the formation of cage-like species. These metallo-cages have application in a wide number of fields including catalysis, substrate encapsulation, sensing, and molecular magnets. While the development of molecular cages continues to expand in all areas, sustainable design has placed a focus on water soluble species, with water being considered a green solvent. Macrocyclic molecules such as cryptands, crown ethers, and calixarenes have been used as host molecules in host-guest chemistry. Here the “host” molecule contains a cavity in which a “guest” molecule can be sequestered. The binding affinity of the host and guest, pore size, and functionality can all affect the final chemistry of the conjoined species.
This research focuses on two primary goals:
1) Developing water soluble base metal capsules for substrate sequestration and sensing, taking advantage of host-guest inclusion properties.
2) Application of water soluble base metal capsule complexes for sustainable cross-coupling reactions.
Lead Researchers:
Stephany Graham, Sam MacDonald, Marissa L. Clapson.
Collaborators:
Justine Guidon, Brian D. Wagner.
Key References:
1. Percástegui, E. G.; Ronson, T. K.; Nitschke, J. R. Design and Applications of Water-Soluble Coordination Cages. Chem. Rev. 2020, 120 (24), 13480–13544.
2. Prochowicz, D.; Kornowicz, A.; Lewiński, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117 (22), 13461–13501.
3. Misener, T. A.; Wagner, B. D. Fluorescence-Based Investigations of the Host–Guest Inclusion of Anilinonaphthalene Sulfonic Acids (1,8- and 2,6-ANS) by Dimethoxypillar[5]Arene in Nonaqueous Solvents. J. Incl. Phenom. Macrocycl. Chem. 2021, 100 (12), 131–141.
4. Xin, X.; Wang, J.; Gong, C.; Xu, H.; Wang, R.; Ji, S.; Dong, H.; Meng, Q.; Zhang, L.; Dai, F.; Sun, D. Cyclodextrin-Based Metal-Organic Nanotube as Fluorescent Probe for Selective Turn-On Detection of Hydrogen Sulfide in Living Cells Based on H2S-Involved Coordination Mechanism. Scientific Reports. 2016, 6 (1), 1–9.
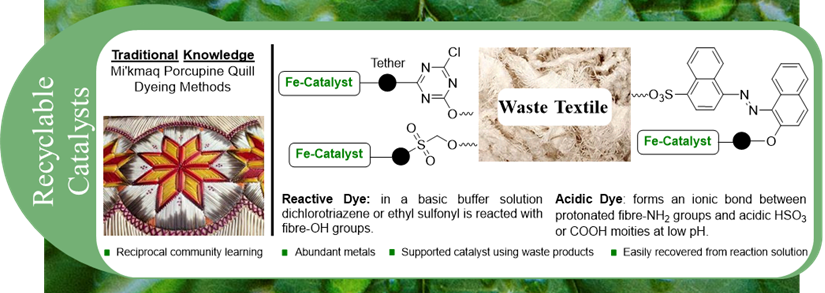
Recyclable Iron Catalysts Via Catalyst Immobilization on Waste Textiles and Keratin
Removal of catalytic metal waste from fine chemical products is of great importance due to their toxicity. Likewise, methods to remove or easily recycle catalytic materials from reaction solutions can help to drive down cost for industrial applications. Catalyst immobilization onto substrates such as nanoparticles, polymers, and carbon-based materials has proven successful in several transformations such as electrocatalytic CO2 reduction, hydroformylation, and C-C cross-coupling with reasonable catalyst recovery. An emerging method in catalyst immobilization is the application of “smart textiles” or “technical textiles”.
This research focuses on two primary goals:
1) Tether iron cross-coupling catalyst to reactive and acidic dye fragments.
2) Explore traditional PEI Mi’kmaq dying methods to create innovative immobilization techniques of catalytic dye fragments onto waste textiles and keratin.
Lead Researchers:
Marissa L. Clapson
Key References:
1. Morshed, M. N.; Behary, N.; Bouazizi, N.; Guan, J.; Nierstrasz, V. A. Chemosphere 2021, 279, 130481.
2. Mayer-Gall, T.; Lee, J. W.; Opwis, K.; List, B.; Gutmann, J. S. ChemCatChem, 2016, 8 (8), 1428–1436.
3. Industrial Dyes: Chemistry, Properties, Applications, 1st ed.; Hunger, K., Ed.; Wiley, 2002.
Active Learning Techniques to Explore Green Chemistry in Inorganic Chemistry Classrooms
Hannah Lawlor, BSc.
Undergraduate Summer Researcher
Project: Realigning Laboratory with Lecture - Updating Inorganic Chemistry I.
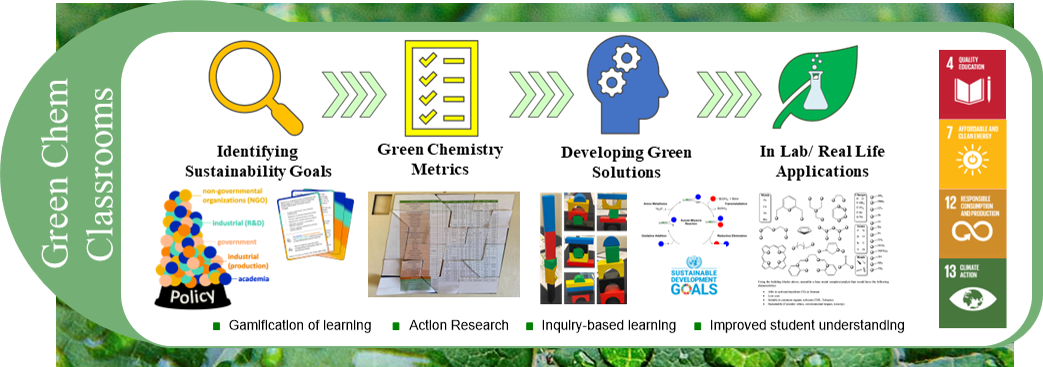
Sustainability and green chemistry principles are at the forefront of modern chemistry research, working to develop chemical processes that focus on bringing the 17 sustainability initiatives developed by the United Nations to fruition. Undergraduate education plays an important role in training and inspiring future researcher, thus, the importance of introducing green chemistry material into all chemistry courses cannot be underestimated. This work focuses on developing connections between green chemistry and inorganic chemistry, working to provide students with a better understanding of sustainability directives as it relates to both chemistry as a whole and catalyst development.
This research focuses on two primary goals:
1) Explore the relationship between green chemistry and inorganic chemistry in undergraduate classrooms
2) Develop active learning and inquiry-based teaching methods to enhance student understanding of undergraduate level inorganic chemistry content.
Lead Researchers:
Hannah Lawlor, Marissa L. Clapson
Key References:
1. Faust, J.L.; Paulson, D. R. Journal of Excellence in College Teaching, 1998, 9, 3.
2. Freeman, S.; Eddy, S. L.; McDonough, M.; Smith, M. K.; Okoroafor, N.; Jordt, H.; Wenderoth, M. Psychological and Cognitive Sciences, 2014, 111, 23, 8410-8415.
3. Deslauriers, L.; McCarty, L. S.; Miller, K.; Callaghan, K.; Kestin, G. PNAS, 2019, 116, 23, 19251-19257.
.